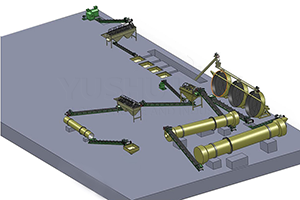
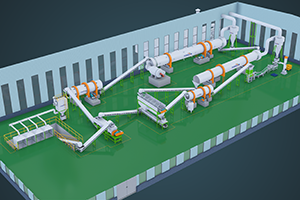
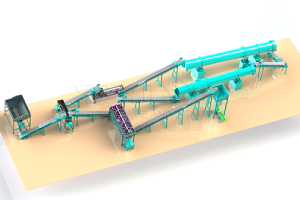
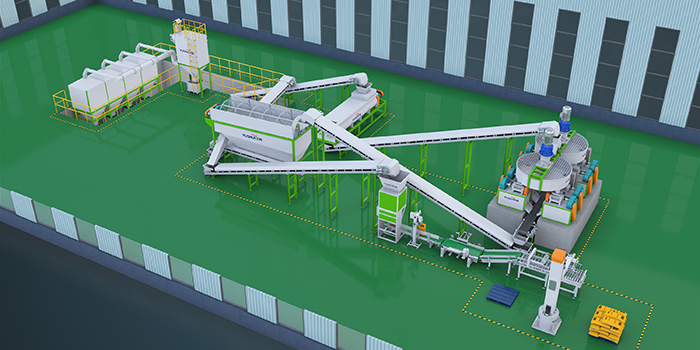
Ammonium sulfate ((Th4)2SO4) - important nitrogen fertilizer, which can be prepared in various ways. Below are some common methods for preparing ammonium sulfate as fertilizer for reference only:
Preparation of ammonium sulfate as a fertilizer by neutralization method:
This is the most commonly used method for producing ammonium sulfate. Ammonium sulfate is obtained by neutralizing ammonia (NH3) or aqueous ammonia (NH4OH) sulfuric acid (H2SO4). Reaction formula:
2NH3+H2SO4→(NH4)2SO4
NH4OH+H2SO4→(Th4)2SO4+H2ONH4OH+H2SO4→(NH4)2SO4+H2O
In industrial production, neutralization reactions are usually carried out in reactors. Ammonium sulfate solution, reaction-formed, Cool, crystallize, centrifuge and they dry to produce solid ammonium sulfate.
By-products law:
Ammonium sulfate is often produced as a by-product of other chemical industrial processes. For example, ammonia gas, formed during the coke production process, may react with sulfuric acid to form ammonium sulfate. Ammonium sulfate is also collected as a by-product in many chemical processes, including pesticide production, fur dyeing and pharmaceuticals.
Method of vapor-phase oxidation of ammonia:
During the production of nitric or sulfuric acid, ammonia gas is mixed with air. Under the influence of a catalyst, ammonia is oxidized to nitrogen oxides (NO or NO2). These nitrogen oxides then react with water to form nitric acid. The reaction of nitric acid with ammonium sulfate produces ammonium nitrate. Ammonium sulfate is then produced through a double displacement reaction with sulfuric acid.
Production of ammonium sulfate as a fertilizer using the sulfuric acid method:
In the production of phosphate fertilizers, phosphoric acid and calcium sulfate (gypsum) obtained by treating phosphate-containing ores with sulfuric acid, eg apatite. If ammonia is added to this process, you can get phosphoric acid and ammonium sulfate. It is then used to prepare ammonium sulfate as a fertilizer.
Urea decomposition method:
Urea can be hydrolyzed under acidic conditions to produce carbon dioxide and ammonia. Ammonia then reacts with sulfuric acid to form ammonium sulfate.
In different industrial processes, you use different methods to produce ammonium sulfate depending on the raw materials available and your needs.. You can contact us. Let us help you choose a method for producing ammonium sulfate as a fertilizer, which makes maximum use of the raw materials used, minimizes costs and has the least impact on the environment.