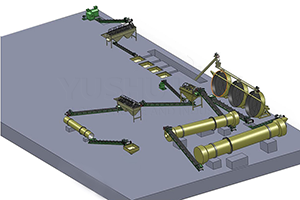
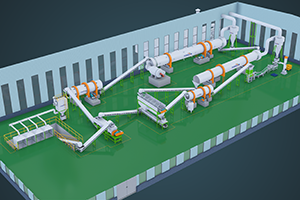
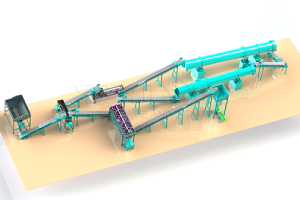
Really, There are several different ones methods for obtaining potassium sulfate (K2SO4). Each of them has its own characteristics and applicable situations. The following information is given about six popular potassium sulfate production processes.
Mannheim skill
This is the traditional process of potassium sulfate. In the Mannheim process, potassium chloride (KCl) and sulfuric acid (H2SO4) react at high temperature in a specially designed furnace. As a result of the reaction, potassium sulfate and gaseous chloride hydrogen are formed (HCl). Chlorovoros can be collected and used for commercial purposes as a by -product. This process requires high energy consumption. And corrosion -resistant equipment is also necessary, capable of resisting high temperatures and aggressive gases.
The process of double decomposition of sulfate and potassium chloride
In this method, potassium sulfate is obtained double decomposition reaction calcium sulfate (CaSO4) and potassium chloride (KCl) In aqueous solution. During the reaction, calcium sulfate is first dissolved in water with the formation of ions. Then he reacts with potassium chloride with the formation of potassium sulfate and calcium chloride (CaCl2). This must usually be done at certain temperatures and concentrations of the solution, to ensure maximum output and cleanliness.
Cooling crystallization method
Cooling crystallization method uses the difference in the solubility of potassium chloride and magnesium sulfate (MgSO4) depending on the temperature. In this method, magnesium sulfate and potassium chloride can be obtained respectively from an unprocessed solution, mined from a mine or salty lake, After a series of stages of evaporation and cooling. Potassium sulfate is separated from the uterine solution by further processing, For example, Crystallization during cooling.
Ion -income method
Method ion exchange - Modern technology for obtaining potassium sulfate. He uses resin or other ion -exchange materials for adsorption of potassium ions from solutions, containing potassium. After saturation of the resin of potassium ions can be eluded from the resin using a concentrated solution of sulfuric acid. Thus receive potassium sulfate. This method allows you to effectively extract potassium from various sources, including wastewater and sea water.
Sulfuric ore burning process
In this process, sulfur -containing ores (For example, pyrite FeS2) mixed with potassium chloride and heat to high temperatures for burning. Sulfuric acid and sulfate gas, the reaction resulting from, can react with potassium chloride with potassium sulfate. This process also solves environmental problems, sulfuric ore. After all, sulfur waste can be turned into healthy chemical products.
Electrolysis method Production of potassium sulfate
Electrolysis produces potassium sulfate by electrolysis of the solution, containing sulfuric acid and potassium chloride. During electrolysis, the water in the solution breaks up into hydrogen ions and oxygen ions. They react on the cathode and anode, respectively. During this process, hydrogen ions can be connected to the ions of sulfate with the formation of potassium sulfate. Oxygen ions can be combined with chloride ions with the formation of gaseous chlorine.
Each method has its own pros and cons of. For example, The Mannheim process is capable of producing potassium sulfate of high purity. However, energy consumption is great, The cost is high, And the requirements for the materials for equipment are high. The double decomposition process is relatively simple. However, by -products may form (such as calcium chloride), which is difficult to handle. The cooling crystallization method is suitable for processing K and MG salts in natural mineral or sea water. The Ion metabolism method and electrolysis method are usually suitable for the development of potassium sources of high purity. If you need additional solutions for the production of potassium sulfate, Please, Contact us!